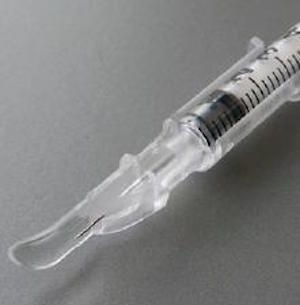
Intradermal Adapter
PATH
Solution Overview & Benefits
Rabies vaccine is expensive and often in short supply—approximately 20,000 people die of rabies each year in India, often because they were unable to access treatment. For rabies vaccine, the same or better protection can be achieved by delivering smaller amounts of vaccine intradermally (into the top layer of the skin), sparing valuable doses and stretching supplies to provide for more patients. Intradermal (ID) regimens for rabies vaccine have been adopted in many regions, protecting people who have been exposed to rabid animals, while saving costs and vaccine.
This approach is not yet universal, despite clear benefits, due in part to the difficulty of giving an ID injection correctly. The Mantoux technique, which uses a traditional needle and syringe for ID delivery, has historically been recognized as a difficult and inconsistently used technique. In many countries, only certain health care workers are able to perform ID injections, such as for rabies vaccine and Bacillus Calmette-Guérin or BCG, a vaccine given intradermally at birth to prevent tuberculosis. Smaller ID doses of other vaccines could also be effective. Such dose sparing could be particularly meaningful to immunization programs in low- and middle-income countries, as they could potentially reduce the amount of vaccine needed per patient, thereby cutting costs and overcoming vaccine shortages. However, concerns regarding the difficulty and a lack of reliability of conventional ID injections limit research in this field and introduction of this technique into immunization programs.
The intradermal adapter makes ID injections easier. It facilitates the procedure by standardizing injection depth and angle. It is anticipated that the ID adapter would expand the pool of health care workers capable of performing the procedure. This would have a particularly high impact in remote or underserved communities with more limited access to health care specialists.
History & Development
In partnership with SID Technologies of Newtown, Pennsylvania, PATH developed the ID adapter from the initial concept through manufacturing of clinic-ready devices. We modified and tested successive design prototypes, incorporating feedback from health care workers in the United States and India. Performance of the current model has been proven in preclinical testing, and will be demonstrated in a clinical trial with saline and ultrasound imaging in the United States in 2011. The ID adapter will then be used in a clinical trial with rabies vaccine in 2011.
Availability
Publicly available: no
Countries where available: contact supplier
Additional Information
Updates
If you are aware of any updates to the Intradermal Adapter project please complete the form or send an email to [email protected]
Need help finding solutions that respond to your unique development challenges? Apply through our Project Accelerator service or Contact us directly for needs-based project consulting.