COVID-19 Rapid Manufacture Ventilator BVM Ambubag
Open Vent - Bristol
Solution Overview & Benefits
Information updated: June 17th 2020
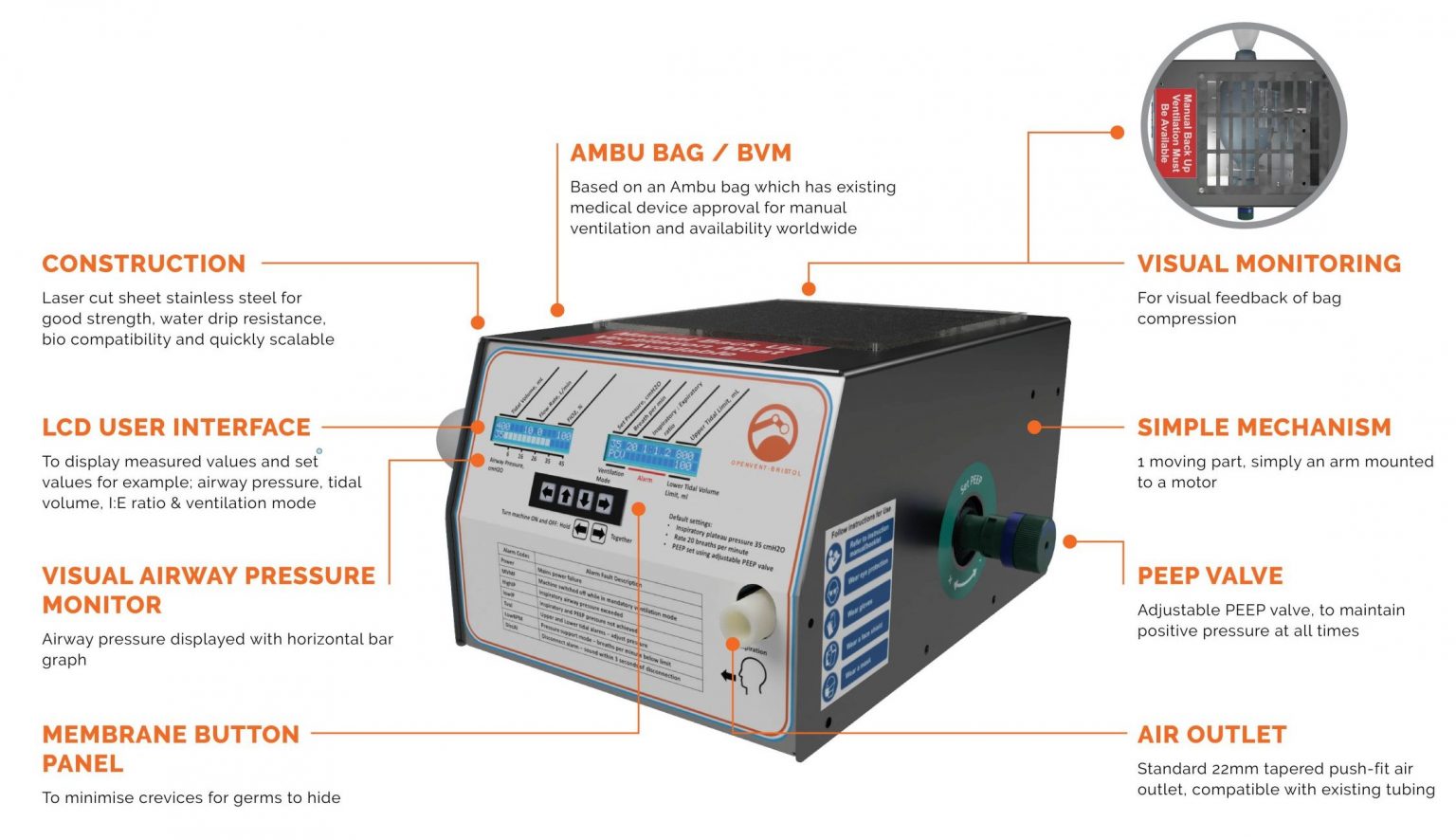
This is an AmbuBag device.
This design is based on a hand-operated BVM (Bag Valve Mask) or known as Ambu bag, which when combined with a PEEP valve will meet most of the NHS requirements for COVID-19 ventilation treatment. The manufacturing process includes laser cutting and using readily available components to ensure low-cost design to be created in bulk.
Spec Summary:
This project has been developed in line with the UK Gov Medicines & Healthcare products
Regulatory Agency Specifications for Rapidly Manufactured Ventilator Systems. It is currently undergoing testing for approval.
(more details can be found on the project websites - see links below)
History & Development
Open Vent Bristol is an open-source initiative led by Darren Lewis. Additional support from Sam Reilly (Software Certification Engineer), Sadie (ITU nurse), Tom Breddal (Consultant Anesthetist in UK ITU). The team are looking for support through their gofundme page.
Team Disclaimer: All users of this design and device shall be deemed notified of the warnings stated herein. This device is a simply designed, fast produced ventilator. This device should not be used in place of an existing hospital ventilator. These should only be used as a last resort where a patient has no other alternative due to the lack of availability of existing ventilators. This is not a fully medically certified device and should not be relied upon as such. The device is designed for use by trained medical professionals and should only be used by trained medical professionals; it is not intended for home use.
The designers and manufacturers of this device shall not be held liable for any death or injury that may result from this device.
The designers and manufacturers of this device give no guarantees or warranties as to the efficacy and/or safety of this device.
This design isn’t connected with Dyson or any other ventilator projects including Dyson CoVent.
THIS DEVICE IS NOT APPROVED FOR CLINICAL USE YET. CURRENTLY UNDERGOING TESTING AT NATIONAL PHYSICAL LAB TO THE MHRA REQUIREMENTS
Availability
Publicly available: No
Countries where available: Developed in the UK but available Worldwide, interest from Saudi Arabia, Ukraine, and Israel to date.
Offered or can be licensed for local manufacture: Yes
Additional Information
Solution Providers Website (external link) Product Webpage (external link)Updates
If you are aware of any updates to the COVID-19 Rapid Manufacture Ventilator BVM Ambubag project please complete the form or send an email to [email protected]
Need help finding solutions that respond to your unique development challenges? Apply through our Project Accelerator service or Contact us directly for needs-based project consulting.